Recognition and Analysis of Pest-Induced Damage in Mangrove Ecosystems: A Case Study in Maros Coastal Region
Abstract
Mangroves in the coastal region of Maros Regency represent a critical ecosystem that plays a vital role in coastal protection, marine habitat provision, and climate change mitigation. However, these mangroves face numerous threats, including pest infestations that can severely damage the ecosystem. This study aims to (1) identify the types of pest attacks and the mangrove species affected in the coastal area of Maros Regency and (2) analyse the level of damage caused by these pest attacks. Using field observation methods, this research provides an in-depth analysis of mangrove damage resulting from pest infestations. The findings reveal several primary types of pest attacks, including leaf suckers, leaf-eating caterpillars, stem borers, and termites. The forms of damage include discoloured leaves, damaged shoots and leaves, open wounds, resin secretion, cracked stems, and broken or dead branches, with the severity ranging from mild to severe, depending on the mangrove species. This data is expected to serve as a foundation for better mangrove management strategies to mitigate pest-induced damage in Maros Regency.
1. Introduction
Mangrove ecosystems are unique coastal habitats with multifunctional roles in maintaining environmental balance and supporting human livelihoods (Das et al., 2022). Acting as natural coastal barriers, mangroves absorb wave energy, prevent erosion, and protect coastal ecosystems from the adverse effects of natural disasters such as tsunamis and storms (Asari et al., 2021; Marois & Mitsch, 2015). Furthermore, mangroves are significant carbon sinks, storing organic carbon in their biomass and soil, and they serve as habitats for various organisms, including fish, shrimp, and mollusks, which enhance local fisheries' productivity (Huxham et al., 2018; Lee et al., 2014).
In the coastal region of Maros Regency, mangrove ecosystems play an essential role not only in environmental protection but also in supporting the livelihoods of coastal communities (Mappiasse et al., 2022; Yusran & Sabar, 2020; Nurrani et al, 2015). However, mangroves in Maros are increasingly threatened by human activities such as waste disposal and logging, particularly in riverine and aquaculture-adjacent areas. These activities have been identified as contributing factors to the decline in mangrove quality (Mappiasse et al., 2022). Additionally, climate change impacts, including sea level rise and saltwater intrusion, exacerbate the degradation of mangrove ecosystems, affecting their ecological functions (de Lacerda et al., 2022).
A significant but often overlooked threat to mangroves is pest infestations. Pest attacks not only reduce the productivity of mangroves but also accelerate their degradation (Utami et al., 2021). Mangrove pests include various species that damage leaves, stems, and roots. Leaf suckers (Homoptera) disrupt photosynthesis, leaf-eating caterpillars (Lepidoptera) cause defoliation, while stem borers (Coleoptera) and root-damaging pests (Isoptera) directly impair the vegetation structure (Marois & Mitsch, 2015; War et al., 2012). These impacts result in stunted growth, reduced biomass, and tree mortality, ultimately disrupting the ecological functions of mangroves (Robertson et al., 1990; Sousa et al., 2003). Unaddressed pest infestations also reduce mangroves’ carbon sequestration capacity, thereby exacerbating global greenhouse gas emissions (Amelia et al., 2023; Ferreira et al., 2024).
In Southeast Asia, mangrove ecosystems face various challenges, including pest and disease damage. However, research on the effects of pest attacks on mangrove health in regions such as Maros Regency remains limited. Studies indicate that biotic stress, such as herbivory and disease, can reduce mangrove growth and hinder their ecological functions, including carbon sequestration (Das et al., 2022; Maldonado-López et al., 2019). Disruptions caused by key functional groups, such as herbivorous crustaceans, can alter ecological processes within mangrove forests (Lee, 1998; Robertson, 1991). Additionally, climate change intensifies these impacts, making mangroves more vulnerable to natural and anthropogenic disturbances (Ellison & Zouh, 2012; Lee et al., 2018). The lack of empirical data on mangrove pests and the extent of damage they cause is a critical barrier to developing effective mitigation strategies. Previous studies in other coastal regions, such as Southeast Asia, have shown that pest damage often goes undetected in its early stages, leading to delayed management responses (Bagarinao & Lantin-Olaguer, 2000; Blasco et al., 2001).
Hence, specific and systematic research on pest identification and their impacts on mangroves in the coastal region of Maros Regency is essential to address this data gap. This study aims to (1) identify the types of pest attacks and the mangrove species affected in the coastal area of Maros Regency and (2) analyse the severity of damage caused by pest infestations. This research is expected to contribute not only to academia but also to policymakers and local communities by providing insights for sustainable mangrove conservation. Such efforts include mangrove restoration, ecological pest control, and increasing public awareness (Osorio et al., 2017; Yeo et al., 2021). Furthermore, the findings aim to inform the development of comprehensive mangrove protection policies at both local and national levels.
Considering the critical importance of mangroves for coastal communities in Maros Regency and the threats they face, this research holds significant value for ensuring the sustainability of mangrove ecosystems.
2. Method
1) Research Location and Time
This study was conducted in the coastal region of Maros Regency over a three-month period in 2023. The observation sites included mangrove areas along the coastline of Maros Regency, located between 4° 53' 1.81" South Latitude and 119° 31' 1.38" East Longitude to 5° 0' 45.70" South Latitude and 119° 28' 33.27" East Longitude (Figure 1).
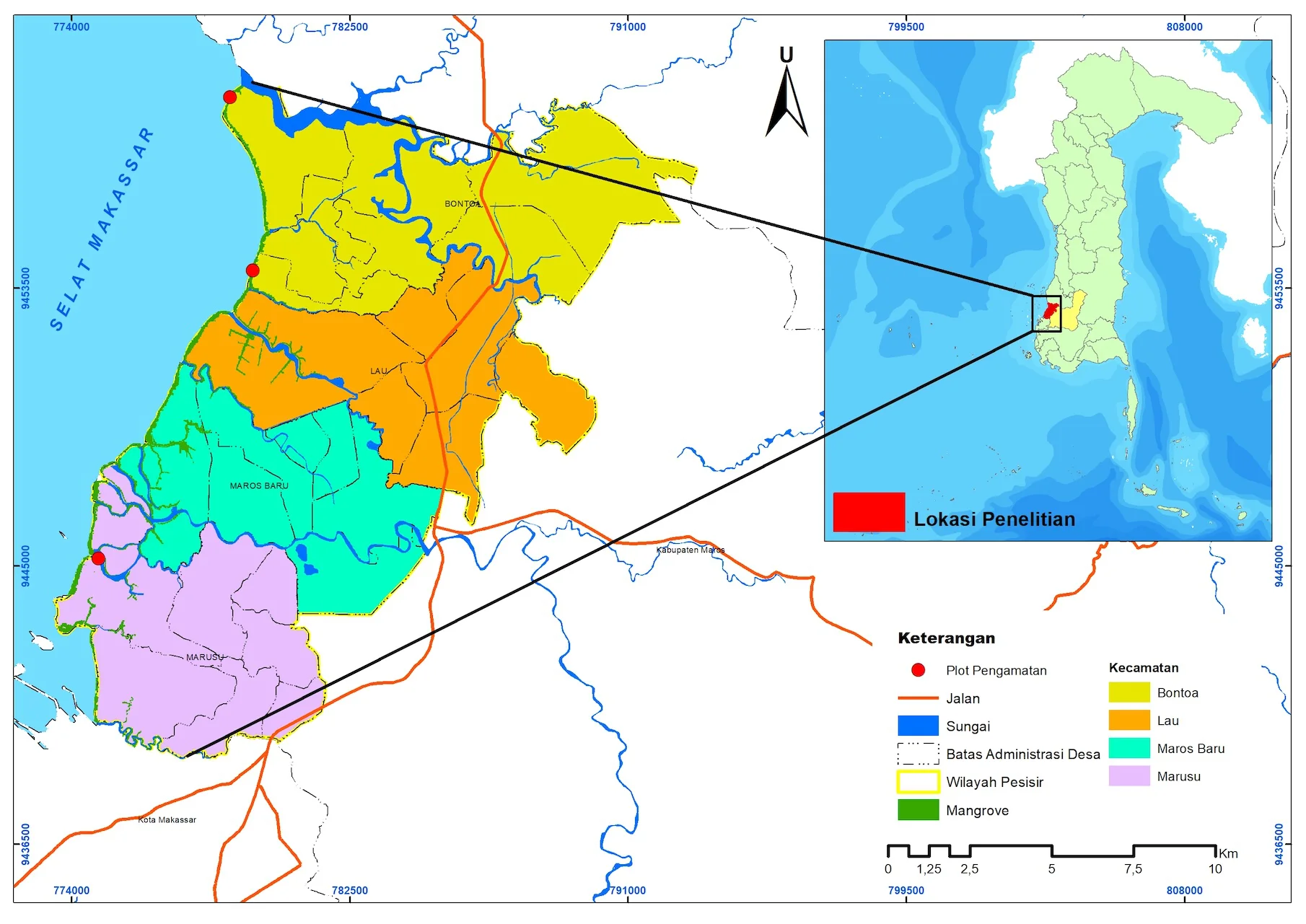
Figure 1. Map of Research Location
The coastal region of Maros Regency, situated in the southern part of Sulawesi, is characterised by its coastline and mangrove ecosystems, which are highly vulnerable to various human activities. These activities include waste disposal near mangrove areas and evidence of mangrove tree destruction, which are indicative of anthropogenic pressures (Mappiasse et al., 2022; Sabar et al, 2023). Additional stressors, such as fishing activities and land conversion for agriculture or settlement, significantly impact the integrity of these ecosystems (Blaber et al., 2000; Miah et al., 2023).
Administratively, the coastal region of Maros Regency spans approximately 30 km along the Makassar Strait. This area is rich in mangrove ecosystems, which are crucial for the preservation of coastal environments. Mangroves provide essential ecosystem services, such as protection against coastal erosion, habitats for various marine species, and significant carbon sequestration, contributing to climate change mitigation (Badwi et al., 2019; Gosari et al., 2024). Maros Regency comprises several districts with direct coastal access, including Marusu District, one of the key coastal areas.
2) Tools and Material
The tools used in this study included a GPS device, a digital camera, tree diameter measuring equipment, a knife, raffia string, a measuring tape, and writing instruments. The materials used consisted of tally sheets and a compilation of literature related to pest infestation indicators and damage patterns in mangroves. These were organised into a field guide to assess mangrove species affected by pests. The classification and types of damage identified included those impacting mangrove leaves, stems, and roots.
3) Research Implementation
Sampling Method
This study employed a purposive sampling method with three observation plots selected for their distinct characteristics. These included mangrove areas dominated by specific mangrove species and those influenced by anthropogenic activities. The locations were chosen based on significant human disturbances and accessibility. The primary rationale for using purposive sampling was to obtain more relevant data on the mangrove ecosystems in the coastal region of Maros Regency, which are potentially exposed to varying environmental threats (Astuti et al., 2018; Perdana et al., 2018; Tjoa & Pattimahu, 2024).
Data Collection Procedure
Observation sites consisted of 20 x 20 metre plots in designated mangrove areas. Within each plot, data collection focused on individual trees, including observations of open wounds, leaf discolouration, damaged shoots and buds, resin secretion, cracked stems, and broken or dead branches. All findings were meticulously recorded on standardised observation sheets and supported with photographic documentation to facilitate visual analysis. The use of observation plots is a standard method in ecosystem research, which not only simplifies monitoring but also ensures consistent and comparable data collection across locations (Madrigal-Martínez & García, 2020; Möller et al., 2009).
4) Data Analysis
Pest Infestation Identification
The identification of pest infestations was conducted within each sampling plot by assigning codes or numbers to ensure that the recorded data could be easily traced. Initial information recorded included the mangrove species and plot location (coordinates or a detailed description). Subsequently, observations of pest attacks were performed on individual mangrove trees by examining physical damage signs on various parts of the trees, such as stems, leaves, shoots, or roots. The method involved visual surveys to document visible damage, including structural or colour changes, and counting the affected parts based on specific parameters, such as damaged leaves or holes in the stems (Bock et al., 2010). Observations were carried out systematically for every tree within the plot and supplemented with additional documentation, such as photographs and field notes, to ensure consistent and accurate data.
This approach aimed to quantitatively measure the extent of damage, providing a clear picture of the impact of pest infestations on specific mangrove species (Jenoh et al., 2016). The observation methods were guided by literature that was compiled to categorise pest types and their attack patterns
Table 1. Presents the assessment of mangrove species based on pest types and the damage indicators observed.
Source: Literature collection results, 2024
Pest Infestation Severity Analysis
To analyse the severity of pest infestations, the observation data were processed through a series of systematic steps. The process began with recording the condition of each individual tree across the three observation plots, including identifying signs of pest attacks on each mangrove tree. The collected data were then grouped by mangrove species to facilitate further analysis.
P = a/b × 100%
Explanation:
P = Infestation percentage
a = Number of infested trees
b = Total number of observed trees
Tabel 2. Criteria for Infestation Severity Catagories

Source: Romadoni et al., (2023) & Wattimena, (2019)
Subsequently, the number of infested trees was calculated and compared to the total number of trees observed in each plot. The severity of the infestation was determined using the pest infestation percentage formula Table 2. The results were classified into five categories: normal, mild, moderate, severe, and very severe. This categorisation was based on the proportion of infested trees relative to the total number of observed individuals, providing an accurate quantitative assessment of the damage caused by pests (Kathiresan & Bingham, 2001; Newkirk & Field, 1991; Wattimena, 2019).
3. Result and Discussion
1) Results of Pest Infestation Type Identification in the Coastal Region of Maros Regency
Field identification revealed that a total of 107 mangrove trees exhibited various types of damage caused by specific pests. The identified pests included leaf suckers (Homoptera), leaf-eating caterpillars (Lepidoptera), stem borers (Coleoptera), and termites (Isoptera). Similar to studies conducted in mangrove areas of South Africa, damage to mangrove trees was attributed to a range of pests, including leaf suckers, leaf-eating caterpillars, stem borers, and termites. In some regions, approximately 45% of mangrove trees were reported to be infested by pests (Osorio et al., 2017)
Table 3. Types of Pests and the Damage Inflicted on Mangroves in the Coastal Region of Maros Regency

Source: Analysis results, 2024
Pest attacks, targeting leaves, stems, and roots, were also observed in the mangroves of the coastal region of Maros Regency. Table 3 provides an overview of the types of pests and the damage they caused to mangroves in this region.
Table 3 presents the damage caused by various pest types to three mangrove species: Rhizophora apiculata, Rhizophora mucronata, and Avicennia lanata. A total of 107 mangrove trees were affected, with Avicennia lanata identified as the most vulnerable species, accounting for 50 impacted trees. Among the identified pests, leaf suckers (Homoptera) infested 12 trees, causing discolouration of leaves, which could reduce photosynthetic efficiency (Choinski et al., 2003; Hu et al., 2020; Kitajima et al., 1997). Similarly, leaf-eating caterpillars (Lepidoptera) attacked 12 trees, primarily targeting shoots and buds. This damage significantly disrupted regeneration and vegetative growth, as shoots are active sites of growth (Kathiresan & Bingham, 2001).
Stem borers (Coleoptera) caused the most severe damage to Avicennia lanata, affecting 11 trees. These trees exhibited open wounds and resin secretion, with four of them showing fractured wood structures. Such damage highlights the significant stress on the vascular tissues of the trees caused by pest attacks. Stem borers bore into stems, creating cavities or holes that compromise the structural integrity of the trees and reduce their ability to sustain mangrove ecosystem functions (Calderón-Cortés et al., 2016; Sousa et al., 2003). Additionally, termites (Isoptera) caused broken or dead branches in 11 trees. The high susceptibility of Avicennia lanata to termite attacks may be attributed to its weaker wood tissue and a lack of natural chemical defences compared to Rhizophora species (Duke & Schmitt, 2015).
These results underscore the varying levels of vulnerability among mangrove species to pest infestations, with Avicennia lanata being particularly susceptible. This highlights the need for species-specific pest management strategies to mitigate damage and maintain the ecological functions of mangrove ecosystems.
The mangrove species Rhizophora apiculata exhibited a relatively high vulnerability to pest infestations, with a total of 36 affected trees. Leaf suckers (Homoptera) were the dominant type of infestation, impacting 13 trees. Symptoms included leaf discolouration, which can reduce photosynthetic efficiency and hinder tree growth (Marois & Mitsch, 2015). Additionally, leaf-eating caterpillars (Lepidoptera) attacked seven trees, causing damage to leaves that could further decrease energy production through photosynthesis (Kathiresan & Bingham, 2001).
Stem borers (Coleoptera) were also observed, with six trees showing open wounds and two trees exhibiting resin secretion (resinosis). While resinosis is a natural defence mechanism in plants, continuous attacks can weaken the trees over time (Luchi et al., 2005). Termite infestations (Isoptera) resulted in broken or dead branches on eight trees. Although Rhizophora apiculata is known for its high tannin content—tannins being compounds with antibacterial and insecticidal properties that can reduce insect attack—termite damage remained a significant threat. Research by Barcoto & Rodrigues (2022) indicates that termites can overcome these defences through their ability to excavate and damage wood tissues.
Furthermore, tannins do not fully protect trees from mechanical damage, such as broken branches, which remain a critical threat, especially for trees already weakened by environmental stressors (Dahdouh-Guebas et al., 2000; Pearce, 1996). Termites are often associated with structural damage or vulnerabilities caused by other factors, such as environmental stress or habitat changes, which reduce a tree’s resistance to attack. This suggests that while tannins may decrease pest attraction, termites can still damage vital tree parts, including branches, ultimately affecting the survival and ecological function of mangrove trees.
In comparison to Rhizophora apiculata, Rhizophora mucronata demonstrated the lowest susceptibility to pest infestations, with only 10 affected trees. Leaf suckers (Homoptera) attacked seven trees, causing symptoms such as changes in leaf shape and colour. Leaf-eating caterpillars (Lepidoptera) impacted only two trees, resulting in minimal leaf damage. Stem borer (Coleoptera) infestations were rare, with just one tree showing signs of open wounds. Notably, no resin secretion (resinosis) was observed in this species, which may indicate that the infestation levels were insufficient to trigger a physiological defence response.
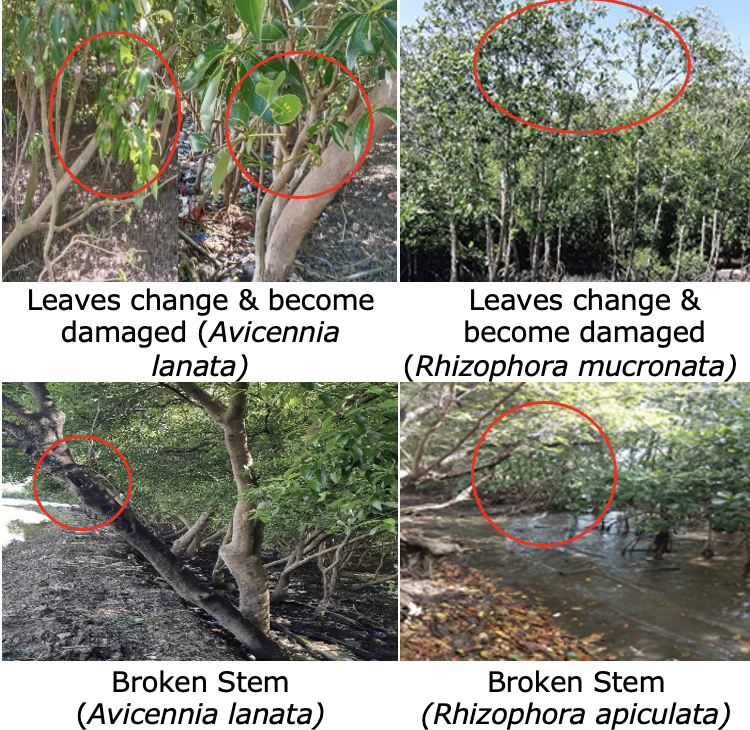
The low infestation rate in Rhizophora mucronata is likely due to its higher tannin content and other chemical defence compounds compared to Avicennia lanata (Chelliah et al., 2023). Tannins, along with other phenolic compounds, act as natural defence mechanisms against insects and pathogens. These compounds possess antibacterial and insecticidal properties that inhibit pest activity (Chelliah et al., 2023; Sadeer et al., 2019). Furthermore, tannins can reduce the availability of nutrients for pests, thereby lowering the likelihood of infestation (Aznawi et al., 2024). Figure 2 provides an illustration of pest infestation symptoms on mangrove trees.
Overall, pests infesting mangrove trees in the coastal region of Maros Regency exhibited diverse damage patterns, ranging from deformed leaves to structural damage to stems and branches. Effective and sustainable pest control measures are essential to maintaining the health of mangrove ecosystems. These measures include managing natural pest habitats, utilising natural predators, and applying biopesticides.
Ineffective or destructive pest control methods can harm mangrove ecosystems and reduce their ecological and socio-economic benefits. Therefore, an integrated approach involving government agencies, local communities, and researchers is crucial for the sustainable management of pest control in mangrove ecosystems (Primavera et al., 2019).
Overall, pests infesting mangrove trees in the coastal region of Maros Regency exhibited diverse damage patterns, ranging from deformed leaves to structural damage to stems and branches. Effective and sustainable pest control measures are essential to maintaining the health of mangrove ecosystems. These measures include managing natural pest habitats, utilising natural predators, and applying biopesticides.

Figure 2. Damage Caused by Pest Infestations
Ineffective or destructive pest control methods can harm mangrove ecosystems and reduce their ecological and socio-economic benefits. Therefore, an integrated approach involving government agencies, local communities, and researchers is crucial for the sustainable management of pest control in mangrove ecosystems (Primavera et al., 2019).
2) Classification of Damage Severity
The damage documented on mangrove trees (Figure 2) highlights the potential for significant impacts on coastal ecosystems. Discoloured and damaged leaves can reduce photosynthetic productivity, which, in turn, hampers the tree's biomass growth. According to Marzuoli et al. (2019), reduced photosynthetic capacity due to leaf damage or discolouration diverts metabolic resources away from growth, resulting in diminished biomass accumulation.
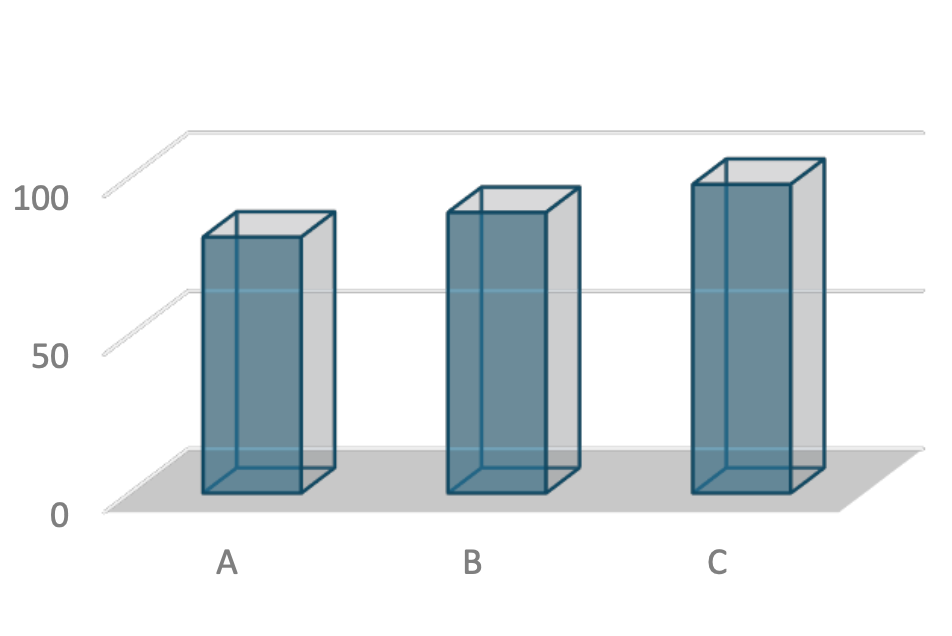
Additionally, pest-induced damage such as open wounds, resin secretion (resinosis), and fractured stems indicates mechanical stress that can weaken tree structures. This increases the risk of tree collapse, particularly during storms or tidal surges. A study by Rimada et al. (2023) revealed that trees with structural defects are more likely to fail under extreme weather conditions, underscoring the importance of effective pest control. Based on the percentage of pest-induced damage (Figure 3), the coastal region of Maros Regency exhibits a range of infestation severity, from low to severe.
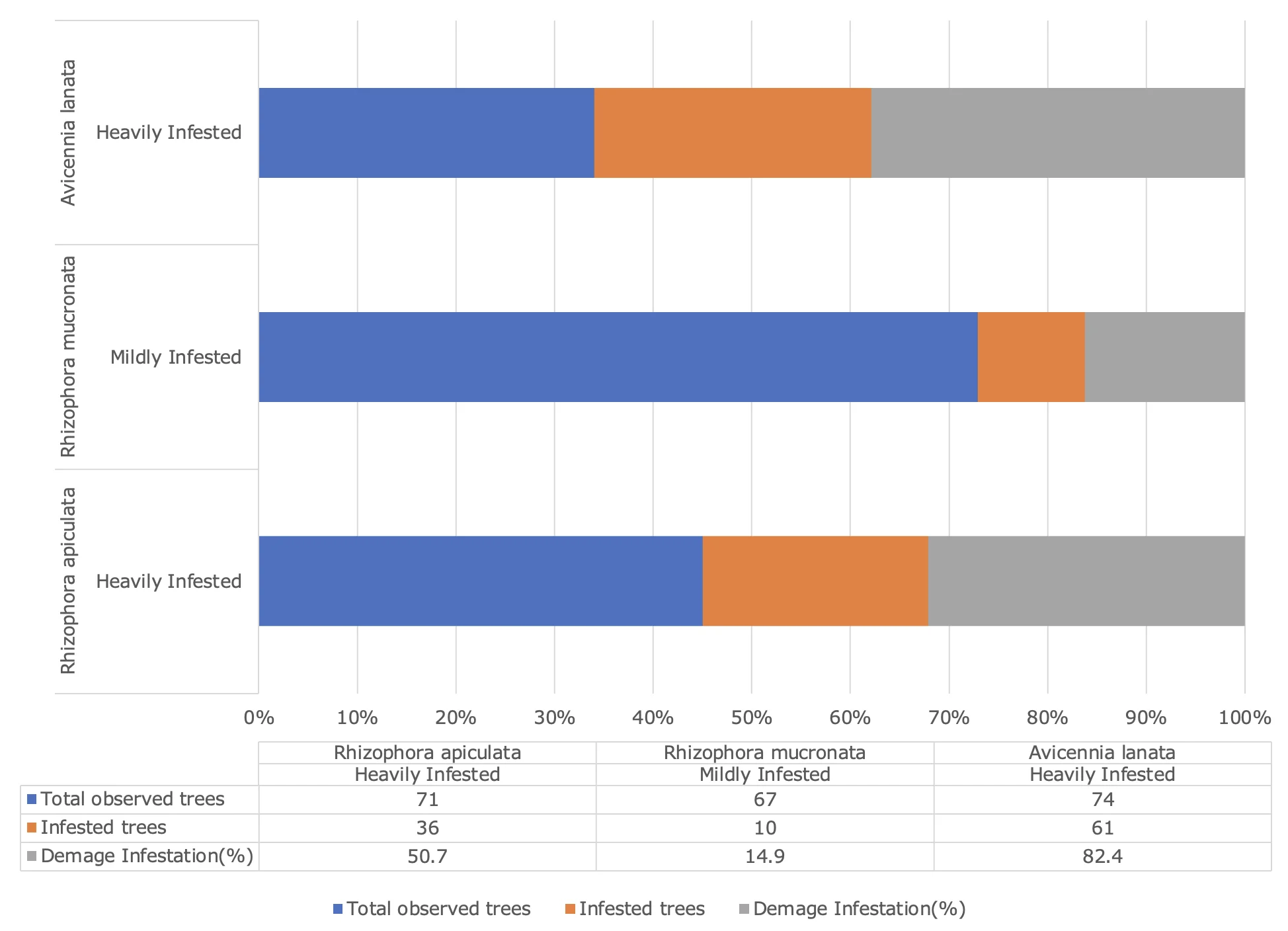
Figure 3. Percentage of Mangrove Pest Damage in the Coastal Region of Maros Regency
The analysis revealed that Rhizophora apiculata experienced heavy pest infestations, with 51% of trees in the observation plots affected. This finding indicates that the species is highly vulnerable to pests such as leaf suckers (Homoptera), leaf-eating caterpillars (Lepidoptera), and stem borers (Coleoptera). A primary factor contributing to this vulnerability is the soft, nutrient-rich leaf structure, which makes it more attractive to herbivorous pests (Kimmerer & Potter, 1987). Damage observed, including leaf discolouration and broken branches, compromises the tree's photosynthetic capacity and structural stability. The high level of infestation may also reflect a lack of natural predators in the area.
In contrast, Rhizophora mucronata exhibited a low level of damage, with only 15% of trees affected. This species appears to possess more effective defence mechanisms against pests, potentially including higher concentrations of secondary compounds in plant tissues or a thicker wax layer on leaves that deters herbivorous insects (Choong et al., 1992). The observed pest activity on this species was limited to leaf suckers and leaf-eating caterpillars, with no significant damage to stems or branches. These results suggest that Rhizophora mucronata is more resilient to pest attacks compared to other species under similar ecological conditions.
For Avicennia lanata, the analysis showed severe damage, with 82% of trees in the observation plots affected. This high percentage indicates that this species is extremely vulnerable to various pests, including leaf-eating caterpillars and stem borers. A key factor contributing to its vulnerability is its habitat, often located in areas with lower salinity and higher exposure, which facilitates pest infestations (Primavera et al., 2019). Damage such as fractured stems and widespread resin secretion can lead to gradual tree mortality, significantly reducing the productivity and ecological functionality of mangrove ecosystems.
The varying levels of pest infestation observed in Rhizophora apiculata, Rhizophora mucronata, and Avicennia lanata highlight the differences in natural resistance and ecological adaptations among these mangrove species. Similarly, Kathiresan and Bingham (2001) noted that pest infestation levels vary across mangrove species due to distinct natural defence mechanisms and adaptive capacities to environmental conditions. These variations are influenced by physical plant structures, the presence of chemical compounds that act as pest deterrents, and the ecological context of their habitats. For instance, Rhizophora mucronata, which exhibited lower levels of damage, may be better adapted to environmental stressors and pest attacks due to features such as its tougher bark, which provides resistance against stem borers (DeYoe et al., 2020). In contrast, Avicennia lanata tends to be more susceptible, as its habitat often provides favourable conditions for pest proliferation. Other factors, such as geographical distribution, soil conditions, and the presence of natural predators, also play significant roles in determining infestation severity (Bhowmik et al., 2022; Tomlinson, 2016).
Pest infestations in mangroves impact not only individual trees but also the broader ecosystem. Pests can disrupt critical ecological functions of mangroves, such as coastal protection, carbon sequestration, and providing habitats for various species. A study conducted in West Kalimantan reported extensive mangrove damage from biotic factors, including termite infestations, leaf-eating caterpillars, and stem borers, which have significantly impaired mangrove ecosystem health in the region (Haneda & Suheri, 2018).
Damage to leaves and branches reduces photosynthetic capacity and biomass production, which, in turn, affects ecosystem functions such as carbon absorption, shoreline stabilisation, and habitat provision for fauna (DeYoe et al., 2020; Kathiresan & Bingham, 2001). The high level of pest infestation observed in Avicennia lanata can accelerate mangrove ecosystem degradation, as this species plays a critical role in stabilising coastal environments. Pests such as stem borers (Coleoptera) and leaf suckers (Homoptera) can inflict severe damage, including fractured stems, discoloured leaves, and premature leaf drop. These damages reduce the tree's ability to photosynthesise, ultimately inhibiting growth and biomass production.
Prolonged pest infestations weaken tree health and may lead to localised tree mortality in mangrove areas. This degradation affects not only individual tree functions but also the entire mangrove ecosystem. Damaged mangrove trees lose their ability to sequester carbon, protect coastlines from erosion, and provide habitats for marine and terrestrial fauna. Over time, ecosystem degradation can increase the risk of saltwater intrusion, biodiversity loss, and disruption of livelihoods for coastal communities (DeYoe et al., 2020; Kathiresan & Bingham, 2001).
4. Conclusion
Based on the research findings, mangroves in the coastal region of Maros Regency face significant pressures from pest infestations. The observed types of damage include leaf discolouration, damage to shoots and buds, open wounds, resin secretion (resinosis), fractured stems, and broken or dead branches. These damages are attributed to pests such as leaf suckers (Homoptera), leaf-eating caterpillars (Lepidoptera), stem borers (Coleoptera), and termites (Isoptera). Among the mangrove species studied, Avicennia lanata exhibited the highest infestation rate (82%), followed by Rhizophora apiculata (51%), with Rhizophora mucronata showing the lowest level of damage (15%).
One key area for further exploration is the specific identification of pest species attacking mangroves. Such identification could provide a clearer understanding of the relationships between pest species and the extent of damage to different mangrove species. Additionally, it is essential to evaluate other environmental factors, such as salinity, water quality, and anthropogenic disturbances, which may influence mangrove susceptibility to pest attacks.
Future research adopting these approaches is expected to yield deeper insights, contributing to the sustainable management and conservation of mangrove ecosystems.
5. Author Contributions
MFM's contribution consists of conceptualization research, design research, data collection, data analysis, interpretation of damage patterns and correction of written language; ANM consists of surveys, field data analysis, data collection, and interpretation of damage patterns.
6. Competing Interests
The authors declare that there are no personal interests or financial relationships that could influence this article.
7. Acknowledgements
We would like to thank the Ministry of Education, Culture, Research and Technology of the Republic of Indonesia for providing grant funding to support this research. Apart from that, we also appreciate the help and support from various parties who have contributed throughout the research process, from start to finish.
8. References
Ahnanto, Syahpirudin, E., Purba Waskita, I., Novita, Hartati, S., Tjala, A., & Zid, M. (2014). Urgensi Pelestarian dan Rehabilitasi Mangrove Bagi Masyarakat Desa Pantai Mekar Kecamatan Muara Gembong. 2. https://www.researchgate.net/profile/Reinardus-Cabuy/publication/342234434_IDENTIFIKASI_KERUSAKAN_AREAL_HUTAN_MANGROVE_AKIBAT_AKTIVITAS_PENDUDUK_DI_DAERAH_AIRTIBA_KABUPATEN_KAIMANA/links/5f7c01ed299bf1b53e10af0e/IDENTIFIKASI-KERUSAKAN-AREAL-HUTAN-MANGROVE
Amelia, R., Basyuni, M., Alfinsyahri, A., Sulistiyono, N., Slamet, B., Bimantara, Y., Harahap, S. S., Harahap, M., Harahap, I. M., Al Mustaniroh, S. S., Sasmito, S. D., & Arifanti, V. B. (2023). Evaluation of Plant Growth and Potential of Carbon Storage in the Restored Mangrove of an Abandoned Pond in Lubuk Kertang, North Sumatra, Indonesia. In Forests (Vol. 14, Issue 1). https://doi.org/10.3390/f14010158
Asari, N., Suratman, M. N., Mohd Ayob, N. A., & Abdul Hamid, N. H. (2021). Mangrove as a Natural Barrier to Environmental Risks and Coastal Protection BT - Mangroves: Ecology, Biodiversity and Management (R. P. Rastogi, M. Phulwaria, & D. K. Gupta (eds.); pp. 305–322). Springer Singapore. https://doi.org/10.1007/978-981-16-2494-0_13
Astuti, S., Muryani, C., & Rindarjono, M. G. (2018). The Community Participation on Mangrove Conservation in Sayung, Demak from 2004-2016. IOP Conference Series: Earth and Environmental Science, 145(1), 12087. https://doi.org/10.1088/1755-1315/145/1/012087
https://doi.org/10.2991/icamr-18.2019.104
Bagarinao, T., & Lantin-Olaguer, I. (2000). From triphenyltins to integrated management of the `pest’ snail Cerithidea cingulata in mangrove-derived milkfish ponds in the Philippines. Hydrobiologia, 437(1), 1–16. https://doi.org/10.1023/A:1026580726648
https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2022.812143
Bhowmik, A. K., Padmanaban, R., Cabral, P., & Romeiras, M. M. (2022). Global Mangrove Deforestation and Its Interacting Social-Ecological Drivers: A Systematic Review and Synthesis. In Sustainability (Vol. 14, Issue 8). https://doi.org/10.3390/su14084433
Blaber, S. J. M., Cyrus, D. P., Albaret, J.-J., Ching, C. V., Day, J. W., Elliott, M., Fonseca, M. S., Hoss, D. E., Orensanz, J., Potter, I. C., & Silvert, W. (2000). Effects of fishing on the structure and functioning of estuarine and nearshore ecosystems. ICES Journal of Marine Science, 57(3), 590–602. https://doi.org/10.1006/jmsc.2000.0723
Blasco, F., Aizpuru, M., & Gers, C. (2001). Depletion of the mangroves of Continental Asia. Wetlands Ecology and Management, 9(3), 255–266. https://doi.org/10.1023/A:1011169025815
Calderón-Cortés, N., Uribe-Mú, C. A., Martínez-Méndez, A. K., Escalera-Vázquez, L. H., Cristobal-Pérez, E. J., García-Oliva, F., & Quesada, M. (2016). Ecosystem engineering and manipulation of host plant tissues by the insect borer Oncideres albomarginata chamela. Journal of Insect Physiology, 84, 128–136. https://doi.org/10.1016/j.jinsphys.2015.10.008
Chelliah, C. K., Murugan, M., Rajivgandhi, G., Gnanasekaran, C., Govindan, R., Maruthupandy, M., Quero, F., Arulraj, A., Viswanathan, M. R., Alharbi, N. S., & Alshammary, N. H. (2023). Phytochemical derivatives and secondary metabolites rich Rhizophora mucronata as an active anti-oxidant and anti-bacterial agent against multi drug resistant bacteria. Journal of King Saud University - Science, 35(8), 102912. https://doi.org/10.1016/j.jksus.2023.102912
Choinski Jr, J. S., Ralph, P., & Eamus, D. (2003). Changes in photosynthesis during leaf expansion in <emph type="2">Corymbia</emph> <emph type="2">gummifera</emph>. Australian Journal of Botany, 51(1), 111–118. https://doi.org/10.1071/BT02008
CHOONG, M. F., LUCAS, P. W., ONG, J. S. Y., PEREIRA, B., TAN, H. T. W., & TURNER, I. M. (1992). Leaf fracture toughness and sclerophylly: their correlations and ecological implications. New Phytologist, 121(4), 597–610. https://doi.org/10.1111/j.1469-8137.1992.tb01131.x
Das, S., & Tah, J. (2022). Mangrove Ecosystems and Their Services BT - Mangroves: Biodiversity, Livelihoods and Conservation (S. C. Das, Pullaiah, & E. C. Ashton (eds.); pp. 139–152). Springer Nature Singapore. https://doi.org/10.1007/978-981-19-0519-3_6 Das, S. K., Patra, J. K., & Thatoi, H. (2016). Antioxidative response to abiotic and biotic stresses in mangrove plants: A review. International Review of Hydrobiology, 101(1–2), 3–19. https://doi.org/10.1002/iroh.201401744
Davidson, T. M., Torchin, M. E., & Smith, C. M. (2023). Introduced mangroves exhibit less leaf damage and greater performance than native mangroves. Biological Invasions, 25(11), 3503–3515. https://doi.org/10.1007/s10530-023-03120-5
de Lacerda, L. D., Ward, R. D., Borges, R., & Ferreira, A. C. (2022). Mangrove Trace Metal Biogeochemistry Response to Global Climate Change. Frontiers in Forests and Global Change, 5. https://doi.org/10.3389/ffgc.2022.817992
DeYoe, H., Lonard, R. I., Judd, F. W., Stalter, R., & Feller, I. (2020). Biological Flora of the Tropical and Subtropical Intertidal Zone: Literature Review for Rhizophora mangle L. Journal of Coastal Research, 36(4), 857–884. https://doi.org/10.2112/JCOASTRES-D-19-00088.1
Duke, N., & Schmitt, K. (2015). Mangroves: Unusual Forests at the Seas Edge (p. pp 1-24). https://doi.org/10.1007/978-3-642-41554-8_129-1
Ellison, J. C., & Zouh, I. (2012). Vulnerability to Climate Change of Mangroves: Assessment from Cameroon, Central Africa. In Biology (Vol. 1, Issue 3, pp. 617–638). https://doi.org/10.3390/biology1030617
Feller, I. C. (1995). Effects of Nutrient Enrichment on Growth and Herbivory of Dwarf Red Mangrove (Rhizophora Mangle). Ecological Monographs, 65(4), 477–505. https://doi.org/10.2307/2963499
Ferreira, A. C., Ashton, E. C., Ward, R. D., Hendy, I., & Lacerda, L. D. (2024). Mangrove Biodiversity and Conservation: Setting Key Functional Groups and Risks of Climate-Induced Functional Disruption. In Diversity (Vol. 16, Issue 7). https://doi.org/10.3390/d16070423
Gosari, B. A. J., Baso, A., Made, S., Amilluddin, Fachri, M. E., Wahid, A., Amri, A., Arief, A. A., Hamzah, Firman, Saru, A., Takril, & Muhtar. (2024). Mangrove Forest Ecosystem in Majene: Hopes or Challenges? IOP Conference Series: Earth and Environmental Science, 1410(1), 12053. https://doi.org/10.1088/1755-1315/1410/1/012053
Haneda, N. F., & Suheri, M. (2018). Hama mangrove di Kecamatan Batu Ampar, Kabupaten Kubu Raya, Kalimantan Barat. Jurnal Silvikultur Tropika, 09(1), 16–23. https://doi.org/10.29244/j-siltrop.9.1.16-23
Hu, W., Lu, Z., Meng, F., Li, X., Cong, R., Ren, T., Sharkey, T. D., & Lu, J. (2020). The reduction in leaf area precedes that in photosynthesis under potassium deficiency: the importance of leaf anatomy. New Phytologist, 227(6), 1749–1763. https://doi.org/10.1111/nph.16644
Huxham, M., Whitlock, D., Githaiga, M., & Dencer-Brown, A. (2018). Carbon in the Coastal Seascape: How Interactions Between Mangrove Forests, Seagrass Meadows and Tidal Marshes Influence Carbon Storage. Current Forestry Reports, 4(2), 101–110. https://doi.org/10.1007/s40725-018-0077-4
Jenoh, E. M., Robert, E. M. R., Lehmann, I., Kioko, E., Bosire, J. O., Ngisiange, N., Dahdouh-Guebas, F., & Koedam, N. (2016). Wide Ranging Insect Infestation of the Pioneer Mangrove Sonneratia alba by Two Insect Species along the Kenyan Coast. PLOS ONE, 11(5), e0154849. https://doi.org/10.1371/journal.pone.0154849
Kathiresan, K., & Bingham, B. L. B. T.-A. in M. B. (2001). Biology of mangroves and mangrove Ecosystems (Vol. 40, pp. 81–251). Academic Press. https://doi.org/10.1016/S0065-2881(01)40003-4
Kimmerer, T. W., & Potter, D. A. (1987). Nutritional quality of specific leaf tissues and selective feeding by a specialist leafminer. Oecologia, 71(4), 548–551. https://doi.org/10.1007/BF00379295
Kitajima, K., Mulkey, S. S., & Wright, S. J. (1997). Decline of photosynthetic capacity with leaf age in relation to leaf longevities for five tropical canopy tree species. American Journal of Botany, 84(5), 702–708. https://doi.org/10.2307/2445906
Lee, C. K. F., Duncan, C., Owen, H. J. F., & Pettorelli, N. (2018). A New Framework to Assess Relative Ecosystem Vulnerability to Climate Change. Conservation Letters, 11(2), e12372. https://doi.org/10.1111/conl.12372
Lee, S. Y. (1998). Ecological role of grapsid crabs in mangrove ecosystems: a review. Marine and Freshwater Research, 49(4), 335–343. https://doi.org/10.1071/MF97179
Lee, S. Y., Primavera, J. H., Dahdouh-Guebas, F., McKee, K., Bosire, J. O., Cannicci, S., Diele, K., Fromard, F., Koedam, N., Marchand, C., Mendelssohn, I., Mukherjee, N., & Record, S. (2014). Ecological role and services of tropical mangrove ecosystems: a reassessment. Global Ecology and Biogeography, 23(7), 726–743. https://doi.org/10.1111/geb.12155
Luchi, N., Ma, R., Capretti, P., & Bonello, P. (2005). Systemic induction of traumatic resin ducts and resin flow in Austrian pine by wounding and inoculation with Sphaeropsis sapinea and Diplodia scrobiculata. Planta, 221(1), 75–84. https://doi.org/10.1007/s00425-004-1414-3
Madrigal-Martínez, S., & Miralles i García, J. L. (2020). Assessment Method and Scale of Observation Influence Ecosystem Service Bundles. In Land (Vol. 9, Issue 10). https://doi.org/10.3390/land9100392
Maldonado-López, Y., Vaca-Sánchez, M. S., Canché-Delgado, A., García-Jaín, S. E., González-Rodríguez, A., Cornelissen, T., & Cuevas-Reyes, P. (2019). Leaf herbivory and fluctuating asymmetry as indicators of mangrove stress. Wetlands Ecology and Management, 27(4), 571–580. https://doi.org/10.1007/s11273-019-09678-z
Mappiasse, M. F., Djafar, M., & Asra, R. (2022). Distribution of mangrove health in the coastal area of Maros Regency in 2021 based on Sentinel-2 satellite imagery. Jurnal Penelitian Kehutanan Wallacea, 11(2), 165. https://doi.org/10.18330/jwallacea.2022.vol11iss2pp165-179
Marois, D. E., & Mitsch, W. J. (2015). Coastal protection from tsunamis and cyclones provided by mangrove wetlands – a review. International Journal of Biodiversity Science, Ecosystem Services & Management, 11(1), 71–83. https://doi.org/10.1080/21513732.2014.997292
Marzuoli, R., Gerosa, G., Bussotti, F., & Pollastrini, M. (2019). Assessing the Impact of Ozone on Forest Trees in An Integrative Perspective: Are Foliar Visible Symptoms Suitable Predictors for Growth Reduction? A Critical Review. In Forests (Vol. 10, Issue 12). https://doi.org/10.3390/f10121144
Miah, M. G., Islam, M. R., Roy, J., Rahman, M. M., & Abdullah, H. M. (2023). A changing coastal ecosystem: Cox’s Bazar in southeastern coastal region of Bangladesh. Environment, Development and Sustainability, 25(7), 6141–6165. https://doi.org/10.1007/s10668-022-02297-4
Möller, T., Kotta, J., & Martin, G. (2009). Effect of observation method on the perception of community structure and water quality in a brackish water ecosystem. Marine Ecology, 30(s1), 105–112. https://doi.org/10.1111/j.1439-0485.2009.00325.x
Nurrani, L. N., Bismark, M. B., & Supratman , S. T. (2015). Institution and Communities Participation in the Conservation Of Mangrove/Case Study in Tiwoho Village, North Province. Jurnal Wasian, 2(1), 21-32. https://doi.org/10.62142/0edd8k42
Osorio, J. A., Crous, C. J., Wingfield, M. J., de Beer, Z. W., & Roux, J. (2017). An assessment of mangrove diseases and pests in South Africa. Forestry: An International Journal of Forest Research, 90(3), 343–358. https://doi.org/10.1093/forestry/cpw063
Perdana, T. A., Suprijanto, J., Pribadi, R., Collet, C. R., & Bailly, D. (2018). Economic valuation of mangrove ecosystem: empirical studies in Timbulsloko Village, Sayung, Demak, Indonesia. IOP Conference Series: Earth and Environmental Science, 139(1), 12035. https://doi.org/10.1088/1755-1315/139/1/012035
Primavera, J. H., Friess, D. A., Van Lavieren, H., & Lee, S. Y. (2019). Chapter 1 - The Mangrove Ecosystem (C. B. T.-W. S. A. E. E. (Second E. Sheppard (ed.); pp. 1–34). Academic Press. https://doi.org/10.1016/B978-0-12-805052-1.00001-2
Rimada, A. S., Coelho Duarte, Ana Paula. Torrano, C., Cazzola, V., Larramendy, P., Silvera, A., Parins, L., Moreira, V., & Perez, E. S. (2023). Fungi associated to Platanus x acerifolia in Uruguay and failure indicators. Agrociencia Uruguay, 27(SE-Plant protection), e989. https://doi.org/10.31285/AGRO.27.989
ROBERTSON, A. I. (1991). Plant-animal interactions and the structure and function of mangrove forest ecosystems. Australian Journal of Ecology, 16(4), 433–443. https://doi.org/10.1111/j.1442-9993.1991.tb01073.x
Robertson, A. I., Giddins, R., & Smith, T. J. (1990). Seed predation by insects in tropical mangrove forests: extent and effects on seed viability and the growth of seedlings. Oecologia, 83(2), 213–219. https://doi.org/10.1007/BF00317755
Romadoni, A. A., Ario, R., & Pratikto, I. (2023). Analisa Kesehatan Mangrove di Kawasan Ujung Piring dan Teluk Awur Menggunakan Sentinel-2A. Journal of Marine Research; Vol 12, No 1 (2023): Journal of Marine ResearchDO - 10.14710/Jmr.V12i1.35040. https://ejournal3.undip.ac.id/index.php/jmr/article/view/35040
Sabar, A., Rusyid, E. I., Diana, F., Ansar, A., Annisa, N., Idzatilangi, W. I., & Agustiningrum, C. (2023). Livelihood Assets of Lantebung Mangrove Ecotourism Community. Jurnal Wasian, 10(02), 01-10. https://doi.org/10.62142/3w65rp54co
Sadeer, N. B., Rocchetti, G., Senizza, B., Montesano, D., Zengin, G., Uysal, A., Jeewon, R., Lucini, L., & Mahomoodally, M. F. (2019). Untargeted Metabolomic Profiling, Multivariate Analysis and Biological Evaluation of the True Mangrove (Rhizophora mucronata Lam.). In Antioxidants (Vol. 8, Issue 10). https://doi.org/10.3390/antiox8100489
Sousa, W. P., Kennedy, P. G., & Mitchell, B. J. (2003). Propagule size and predispersal damage by insects affect establishment and early growth of mangrove seedlings. Oecologia, 135(4), 564–575. https://doi.org/10.1007/s00442-003-1237-0
Sousa, W. P., Quek, S. P., & Mitchell, B. J. (2003). Regeneration of Rhizophora mangle in a Caribbean mangrove forest: interacting effects of canopy disturbance and a stem-boring beetle. Oecologia, 137(3), 436–445. https://doi.org/10.1007/s00442-003-1350-0
Thu, P. Q., Quang, D. N., Chi, N. M., Hung, T. X., Binh, L. V, & Dell, B. (2021). New and Emerging Insect Pest and Disease Threats to Forest Plantations in Vietnam. In Forests (Vol. 12, Issue 10). https://doi.org/10.3390/f12101301
Tjoa., M., & Pattimahu., D. (2024). PARTISIPASI MASYARAKAT DALAM PENGELOLAAN MANGROVE DI DESA WAIHERU KECAMATAN BAGUALA KOTA AMBON. MARSEGU : Jurnal Sains Dan Teknologi, 1(5 SE-Articles), 452–466. https://doi.org/10.69840/marsegu/1.5.2024.452-466
Tomlinson, P. B. (2016). The Botany of Mangroves. Cambridge University Press. https://books.google.co.id/books?id=vYswDQAAQBAJ
Utami, S., Kunarso, A., Kurniawan, A., Lelana, N. E., & Haneda, N. F. (2021). Pests of Sonneratia caseolaris seedlings in the mangrove restoration area nursery of Berbak-Sembilang National Park and its damage. IOP Conference Series: Earth and Environmental Science, 914(1), 12019. https://doi.org/10.1088/1755-1315/914/1/012019
War, A. R., Paulraj, M. G., Ahmad, T., Buhroo, A. A., Hussain, B., Ignacimuthu, S., & Sharma, H. C. (2012). Mechanisms of plant defense against insect herbivores. Plant Signaling & Behavior, 7(10), 1306–1320. https://doi.org/10.4161/psb.21663
Wattimena, C. M. A. (2019). Serangan Hama Penggerek Daun Pada Tegakan Damar (Agathis Alba) Di Negeri Hunitetu, Kecamatan Inamosol, Kabupaten Seram Bagian Barat. Jurnal Nusa Sylva, 18(1), 17–22. http://ejournalunb.ac.id/index.php/JNS/article/view/211
Yeo, D., Srivathsan, A., Puniamoorthy, J., Maosheng, F., Grootaert, P., Chan, L., Guénard, B., Damken, C., Wahab, R. A., Yuchen, A., & Meier, R. (2021). Mangroves are an overlooked hotspot of insect diversity despite low plant diversity. BMC Biology, 19(1), 202. https://doi.org/10.1186/s12915-021-01088-z
Yusran, & Sabar, A. (2020). Mangrove management collaboration in the Marusu coastal region of Maros regency. IOP Conference Series: Earth and Environmental Science, 486(1), 12036. https://doi.org/10.1088/1755-1315/486/1/012036
Copyright (c) 2024 Jurnal Wasian

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright and License
All articles published in Wasian Journal are the property of the authors. By submitting an article to Wasian Journal, authors agree to the following terms:
-
Copyright Ownership: The author(s) retain copyright and full publishing rights without restrictions. Authors grant the journal the right to publish the work first and to distribute it as open access under a Creative Commons Attribution 4.0 International License (CC BY 4.0).
-
Licensing: Articles published in Wasian Journal are licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0). This license allows others to share, copy, and redistribute the material in any medium or format, and adapt, remix, transform, and build upon the material for any purpose, even commercially, provided that proper credit is given to the original author(s) and the source of the material

This work is licensed under a Creative Commons Attribution 4.0 International License. -
Author's Rights: Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges and greater citation of published work.
-
Third-Party Content: If your article contains material (e.g., images, tables, or figures) for which you do not hold copyright, you must obtain permission from the copyright holder to use the material in your article. This permission must include the right for you to grant the journal the rights described above.
-
Reprints and Distribution: Authors have the right to distribute the final published version of their work (e.g., post it to an institutional repository or publish it in a book), provided that the original publication in Wasian Journal is acknowledged.
For the reader you are free to:
- Share — copy and redistribute the material in any medium or format for any purpose, even commercially.
- Adapt — remix, transform, and build upon the material for any purpose, even commercially.
- The licensor cannot revoke these freedoms as long as you follow the license terms.
Under the following terms:
- Attribution — You must give appropriate credit , provide a link to the license, and indicate if changes were made . You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use.
- No additional restrictions — You may not apply legal terms or technological measures that legally restrict others from doing anything the license permits.
Notices:
You do not have to comply with the license for elements of the material in the public domain or where your use is permitted by an applicable exception or limitation .
No warranties are given. The license may not give you all of the permissions necessary for your intended use. For example, other rights such as publicity, privacy, or moral rightsmay limit how you use the material.